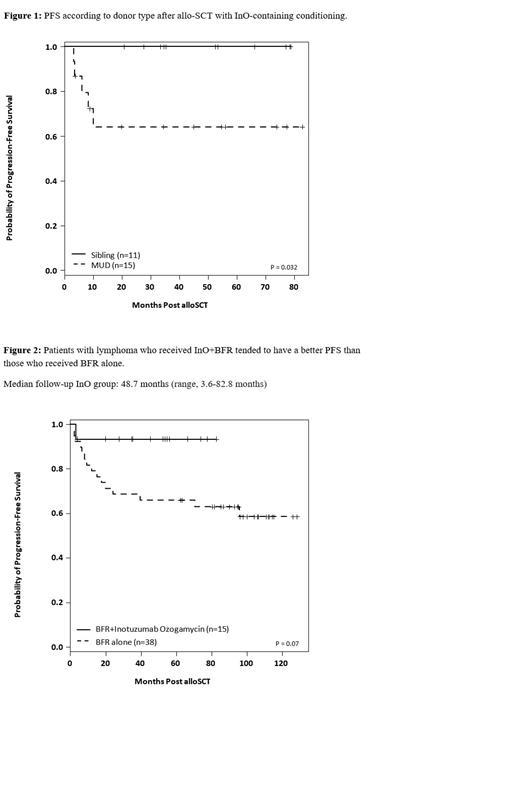
Background: Inotuzumab ozogamicin (InO) is a humanized antibody-drug conjugate that targets CD22+ B-cells. InO demonstrated antitumor activity and manageable toxicity in phase 1/2 trials for the treatment of B-cell non-Hodgkin lymphoma (NHL) as a single agent and in combination with rituximab. In order to improve outcomes in patients with relapsed CD22 (+) NHL, or chronic lymphocytic leukemia (CLL) who failed targeted therapies and were candidates for allo-SCT, we prospectively studied the addition of InO to our standard chemo-conditioning of BFR (bendamustine, fludarabine and rituximab-Khouri Blood 2014). Herein we report long-term survival outcomes. Methods: InO was infused intravenously (iv) on day -13 outpatient, with a dose cohort of 0.6, 1.2 or 1.8 mg/m2. Bendamustine 130 mg/m 2 iv daily on days -5 to -3 together with 30 mg/m 2 iv of fludarabine on days -5 to -3 were given prior to transplantation. Rituximab was given at a dose of 375 mg/m 2 iv on days -6, +1, and +8. Tacrolimus and mini-methotrexate were used for graft versus host disease (GVHD) prophylaxis. Results: The study included 26 patients. Median age was 59 (range, 26-70) years. Disease types: CLL [n=11 (27%) ; 64% with TP53 mutations (either alone, or with other mutations such as BTK, CDH2, ZMYM3); 22% with complex cytogenetics; 75% with unmutated IGHV; mantle cell lymphoma ( MCL) [n=8 (31%); 83% had Ki 67 >30%; 25% TP53 mutation; and 25% blastoid histology]; Follicularlymphoma (n=5, 19%), and diffuse large b cell ( DLBCL[n= 2; (8%)]. Median # prior treatments was 2.5 (range, 1-6). Patients with CLL/MCL were previously treated with ibritunib (n=10), venetoclax (n=5), idelalisib (n=2), nivolumab (n=1) and CAR T (n=1). At study entry, 18 (69%) patients were in CR, 7 (27%) in PR, and 1 (4%) had SD. Eleven (42%) received their transplants from matched sibling donors (MSDs) and 15 (58%) from matched unrelated donors (MUDs). The number of patients who received the 0.6, 1.2 or 1.8 mg/m2 of InO were 4, 2 and 20 patients, respectively. No DLT was observed. Forty-two percent of patients never experienced severe neutropenia and 77% never experienced platelet counts < 20K x 10 9/L. Only 1 patient developed veno-occlusive disease, confounded by the simultaneous manisfestation of hyper-acute GVHD related to prior nivolumab pre-alloSCT. Treatment-related mortality (TRM) at 2-years was 12%. With a median follow-up of 48.7 months (range, 3.6-82.8), the 5-year overall survival (OS) and progression-free survival rates (PFS) were 84% and 80%, respectively. Seven of 8 (87.5%) patients with PR/SD at study entry converted to CR after allo-SCT. There was no significant difference in OS or PFS by histology subtype. Patients who received a transplant from MSDs had OS and PFS rates of 100% v 79% ( P = .060) and 64% ( P = .032) for those who received MUDs, respectively (Figure 1). We compared results of this trial to a group of patients (n=56) with relapsed lymphoid malignancies who received allo-SCT at our center in a preceding prospective trial using BFR conditioning without InO and the same GVHD prophylaxis (clinicaltrials.gov #NCT00880815) which was previously published. There was no statistically significant difference in patients, disease (including histology, disease status pre-transplant) or transplant characteristics between the 2 groups. We found no statistically significant differences in engraftment times, incidence and grades of liver toxicity, TRM, risk of acute grade II-IV or III-IV GVHD. However, the study group containing InO had a higher incidence of extensive chronic GVHD (mainly de novo) than the control group (50% vs 25%, P = .019), respectively. There was a trend in patients with NHL to have a better 5-year OS (93% vs 68%) and 5-year PFS (93% vs 58%, Figure 2) in the study group vs the control groups. We did not observe such a trend in patients with CLL (5-year PFS 62% vs 59%): this could be related to small #patients, level of expression of CD22, more adverse mutations in the study group.
Conclusions: Our results show that InO is safe when combined with an allo-SCT conditioning regimen and may improve survival outcomes in patients with CD22 (+) NHL. This needs to be validated in a larger number of patients. An ongoing trial at our center involves fractionating InO dose pre-and post-allo-SCT in patients with lymphoma or acute lymphoblastic leukemia receiving a reduced-intensity conditioning, and adding post-transplant cyclophosphamide to decrease the risk of GVHD.
OffLabel Disclosure:
Khouri:Pfizer: Research Funding. Jain:Genentech: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Novalgen: Research Funding; Newave: Research Funding; Loxo Oncology: Research Funding; ADC Therapeutics: Research Funding; Kite/Gilead: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Dialectic Therapeutics: Research Funding; Cellectis: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Precision Biosciences: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Beigene: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Medisix: Research Funding; Takeda: Research Funding; Servier: Research Funding; Aprea Therapeutics: Research Funding; Incyte: Research Funding; MEI Pharma: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES; Ipsen: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES; TG Therapeutics: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; BMS: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Pfizer: Research Funding; Mingsight: Research Funding; Fate Therapeutics: Research Funding; CareDX: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; TransThera Sciences: Research Funding; Janssen: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; AstraZeneca: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Pharmacyclics: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; AbbVie: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding. Short:Stemline therapeutics: Research Funding; Amgen: Honoraria; Novartis: Consultancy; Pfizer: Consultancy; Takeda: Consultancy, Research Funding; Astellas: Research Funding; AstraZeneca: Consultancy. Kadia:Janssen Research and Development: Research Funding; Liberum: Consultancy; Novartis: Consultancy; Genentech: Consultancy, Research Funding; GenFleet Therapeutics: Research Funding; AbbVie, Amgen, Inc, Ascentage Pharma Group, Astellas Pharma Global Development, Astex, AstraZeneca, BMS, Celgene, Cellenkos Inc, Cyclacel, Delta-Fly Pharma, Inc, Genentech, Inc., Genfleet, Glycomimetics, Iterion, Janssen Research and Development: Research Funding; Daiichi Sankyo, Genentech, Inc., Genzyme, Jazz Pharmaceuticals, Liberum, Novartis, Pfizer, PinotBio, Inc, Pulmotect, Inc, Sanofi-Aventis, Servier: Consultancy; Iterion: Research Funding; Pulmotect, Inc.: Consultancy, Research Funding; Cure: Speakers Bureau; Delta-Fly Pharma, Inc.: Research Funding; Cyclacel: Research Funding; Jazz Pharmaceuticals, Pfizer, Pulmotect, Inc, Regeneron Pharmaceuticals, SELLAS Life Sciences Group: Research Funding; Sanofi-Aventis: Consultancy; Regeneron Pharmaceuticals: Research Funding; Pfizer: Consultancy, Research Funding; SELLAS Life Sciences Group: Research Funding; Celgene: Research Funding; Cellenkos Inc.: Research Funding; AstraZeneca: Research Funding; Biologix, Cure, Hikma Pharmaceuticals: Speakers Bureau; Hikma Pharmaceuticals: Speakers Bureau; Glycomimetics: Research Funding; Genzyme: Honoraria; Amgen, Inc.: Research Funding; Astellas Pharma Global Development: Research Funding; Ascentage Pharma Group: Research Funding; Agios: Consultancy; Servier: Consultancy; Pinotb-Bio: Consultancy; BMS: Consultancy, Research Funding; Astex: Honoraria. Daher, MD:Takeda: Patents & Royalties. Rafei:Takeda: Other: H. R. and The University of Texas MD Anderson Cancer Center have an institutional financial conflict of interest with Takeda Pharmaceutical. . Marin:Affimed: Patents & Royalties; Takeda: Patents & Royalties. Qazilbash:Bioline: Other: Advisory board; Amgen: Research Funding; Janssen: Research Funding; Angiocrine: Research Funding; NexImmune: Research Funding. Srour:Orca Bio: Research Funding. Kebriaei:Pfizer: Consultancy, Honoraria; Jazz: Consultancy, Honoraria. Shpall:Affimed: Other: License agreement; Axio: Membership on an entity's Board of Directors or advisory committees; Takeda: Other: License agreement; Fibrobiologics: Membership on an entity's Board of Directors or advisory committees; Celaid Therapeutics: Membership on an entity's Board of Directors or advisory committees; Navan: Membership on an entity's Board of Directors or advisory committees; Adaptimmune: Membership on an entity's Board of Directors or advisory committees; NY Blood Center: Membership on an entity's Board of Directors or advisory committees; Syena: Other: License agreement. Kantarjian:Amgen (Inst): Research Funding; Precision Biosciences: Honoraria; Shenzhen Target Rx: Honoraria; Taiho Pharmaceutical: Honoraria; Ascentage Pharma Group: Honoraria; KAHR Medical: Honoraria; Jazz Pharmaceuticals (Inst): Honoraria, Research Funding; Amgen: Honoraria; Bristol-Myers Squibb (Inst): Research Funding; Ascentage Pharma (Inst): Research Funding; Novartis (Inst): Research Funding; Abbvie (Inst): Research Funding; Ipsen: Honoraria; Astellas Pharma: Honoraria; AstraZeneca/MedImmune: Honoraria; Daiichih-Sankyo (Inst): Honoraria, Research Funding; Immunogen (Inst): Honoraria, Research Funding; Novartis: Honoraria; Pfizer: Honoraria; Abbvie: Consultancy, Honoraria. Champlin:Johnson & Johnson/Janssen: Consultancy; Actinium Pharmaceuticals: Consultancy; Omeros: Consultancy; Cell Source: Research Funding; Takeda Corporation: Patents & Royalties; Orca Bio: Consultancy; Arog: Consultancy; Kadmon: Consultancy. Jabbour:Takeda: Consultancy, Honoraria, Research Funding; Ascentage Pharma Group: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Adaptive Biotech: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Hikma Pharmaceuticals: Consultancy, Honoraria, Research Funding.
inotuzumab ozogamicin in allogeneic transplantation

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal